Vaccines against the coronavirus SARS-CoV-2 have been given to billions of people to protect them from COVID-19, and have saved more than 20 million lives. But viral variants can evade some of the immunity provided by the original vaccines. As a result, vaccine developers around the world are working on dozens of ‘next-generation’ COVID-19 vaccines: not just updates of the first versions, but ones that use new technologies and platforms.
These vaccines are a diverse group, but the overarching aim is to deliver long-lasting protection that is resilient to viral change. Some could protect against broader classes of coronavirus, including ones that have yet to emerge. Others might provide more potent immunity, might do so at lower doses, or might be better at preventing infection or transmission of the virus.
Here’s what to expect of this next generation of vaccines.
Why do we need more vaccines?
In a word: evolution. The first approved COVID-19 vaccines were tested for protection against versions of SARS-CoV-2 that had not changed much since the virus was first identified. These vaccines come in different types — some are composed of messenger RNA, others are inactivated versions of the coronavirus itself or some of its proteins — but all work by exposing the body to antigens (portions of the virus) to provoke an immune response without causing disease.
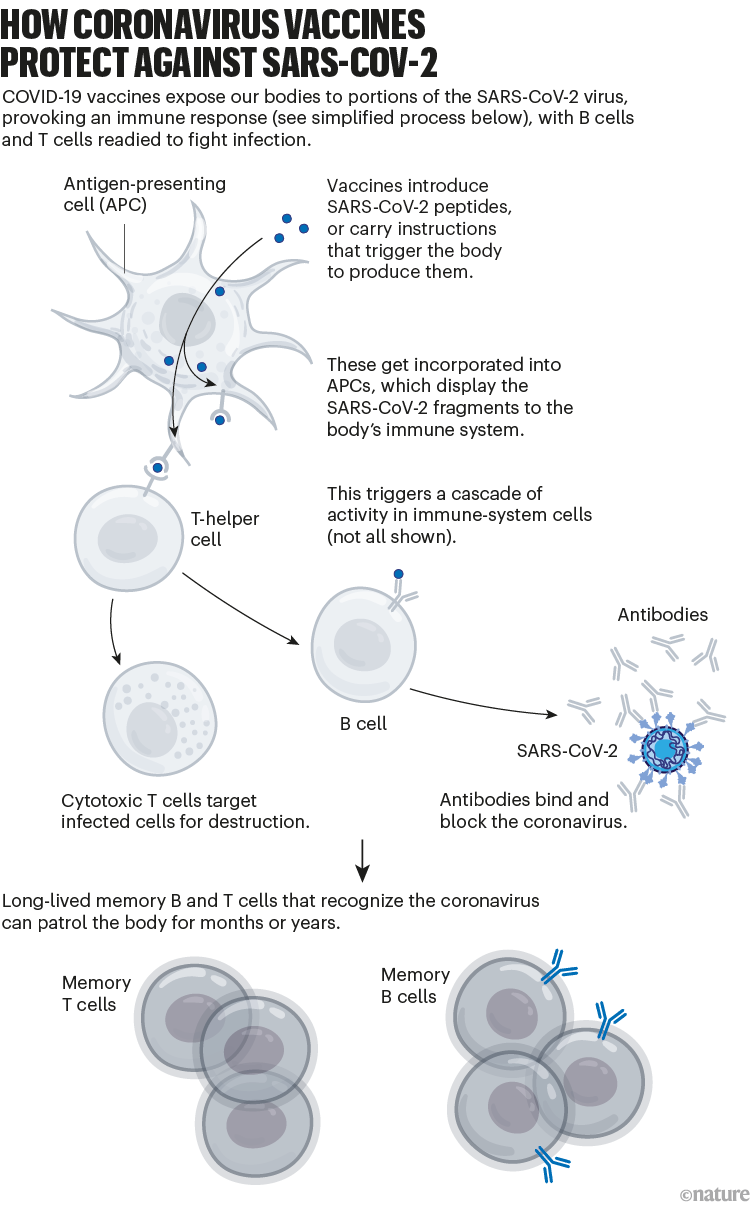
Broadly speaking, this immune response comes from B cells, which produce antibodies that can block SARS-CoV-2 from infecting cells, and from T cells, which can destroy infected cells (and support other immune responses).
The vaccinations also generate a pool of ‘memory cells’ for prolonged immunity, even after initial antibody levels dwindle. On subsequent infection, memory B cells begin proliferating and differentiating into cells that churn out more antibodies (see ‘How coronavirus vaccines protect against SARS-CoV-2’).
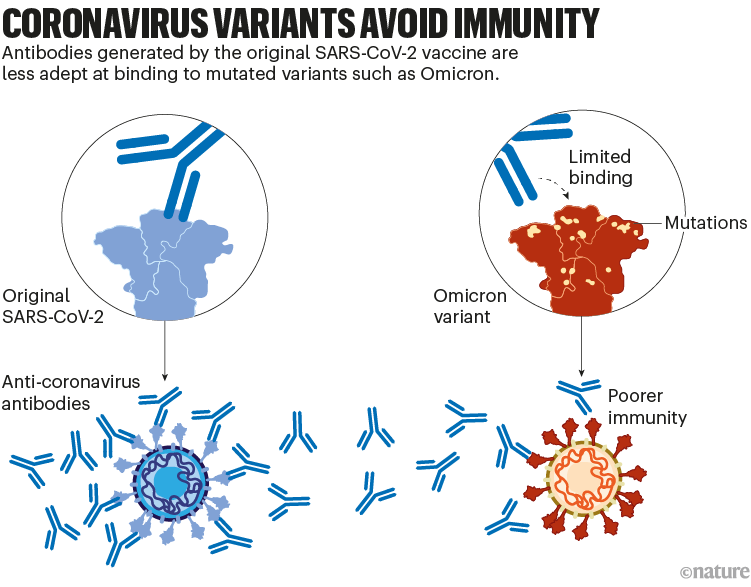
Although these vaccines provide long-lasting protection against severe disease, the protection they offer against viral infection dwindles in months. And variants of SARS-CoV-2, such as Omicron, have since evolved with mutations that allow them to escape some of this immunity. For instance, memory responses generated by the initial vaccines produce antibodies that don’t latch on to Omicron as easily. That contributes to the reduced protection against infection (see ‘Coronavirus variants avoid immunity’).
A second generation of vaccines has already been introduced to boost immunity against the Omicron variant. It’s likely that further, variant-specific updates to vaccines will follow, to try to keep up with viral evolution — although it’s not clear whether the protection they offer will be particularly long-lasting as immunity wanes and SARS-CoV-2 evolves further.
As a result, research teams are taking several approaches to develop new vaccines.
Updated vaccines
To tackle SARS-CoV-2 variants, the vaccine developers Pfizer–BioNTech and Moderna introduced updated mRNA vaccines last year. These are called bivalent, because they encode molecules of the spike protein from the original virus and from Omicron. (The spike protein is what SARS-CoV-2 uses to bind to cells.)
The bivalent vaccines work in several ways. Like other COVID-19 booster shots, they stimulate the memory B cells already established by previous vaccines; some of this cellular response leads to antibodies that can recognize Omicron. Their potency can strengthen over time, too: when presented with Omicron’s spike, memory B cells go through an evolutionary ‘training’ process of mutation and selection, producing a pool of B cells that encode antibodies that bind more tightly to Omicron’s spike. Finally, the Omicron components of bivalent vaccines also recruit new B cells that produce their own antibodies (see ‘Updated vaccines’).
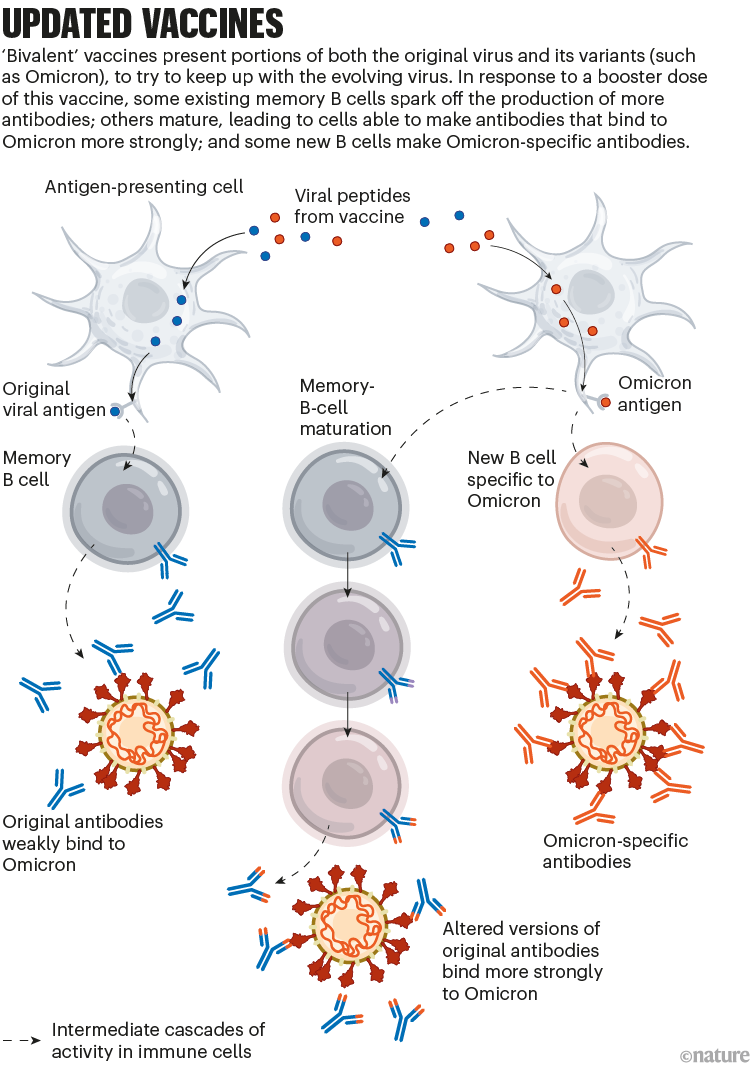
These effects might mean that a bivalent booster provides better protection against Omicron than does a booster dose of the original vaccine. But it’s still unclear how substantial that advantage is in practice.
Some developers, including Pfizer–BioNTech, are also working on combination vaccines to protect people against COVID-19 and other diseases — most commonly influenza. Nearly all are in the early stages of development.
Broadly protective vaccines
Updates to COVID-19 vaccines will always be a step or two behind the evolving virus. Scientists hope to develop ‘broadly protective’ vaccines that can target future SARS-CoV-2 variants — and even related coronaviruses.
The goal of some of these vaccines is to generate an immune response against particular regions of the spike protein that are conserved across SARS-CoV-2 variants and some related coronavirus species, meaning that they tend not to mutate in new variants. One region of interest is the receptor-binding domain (RBD), which binds to the ACE2 receptor protein on human cells and is targeted by some of the body’s most potent infection-blocking antibodies.
At least two teams, at the University of Washington in Seattle and at the California Institute of Technology (Caltech) in Pasadena, are making ‘mosaic’ vaccines: nanoparticles dotted with RBDs from SARS-CoV-2 and coronaviruses from the same family (called sarbecoviruses), such as SARS-CoV and others isolated from bats.
When a B cell recognizes more than one RBD on these mosaic nanoparticles — latching on to conserved regions from multiple virus species — it binds strongly. This, in turn, triggers that B cell to multiply and produce more antibodies (as well as memory B cells to fight future infections). B cells that recognize an RBD from just one viral species bind weakly, and do not generate this response. Researchers hope that using mosaic nanoparticles will result in an enriched pool of antibodies that can recognize multiple RBDs across coronavirus species (see ‘Broader protection?’).
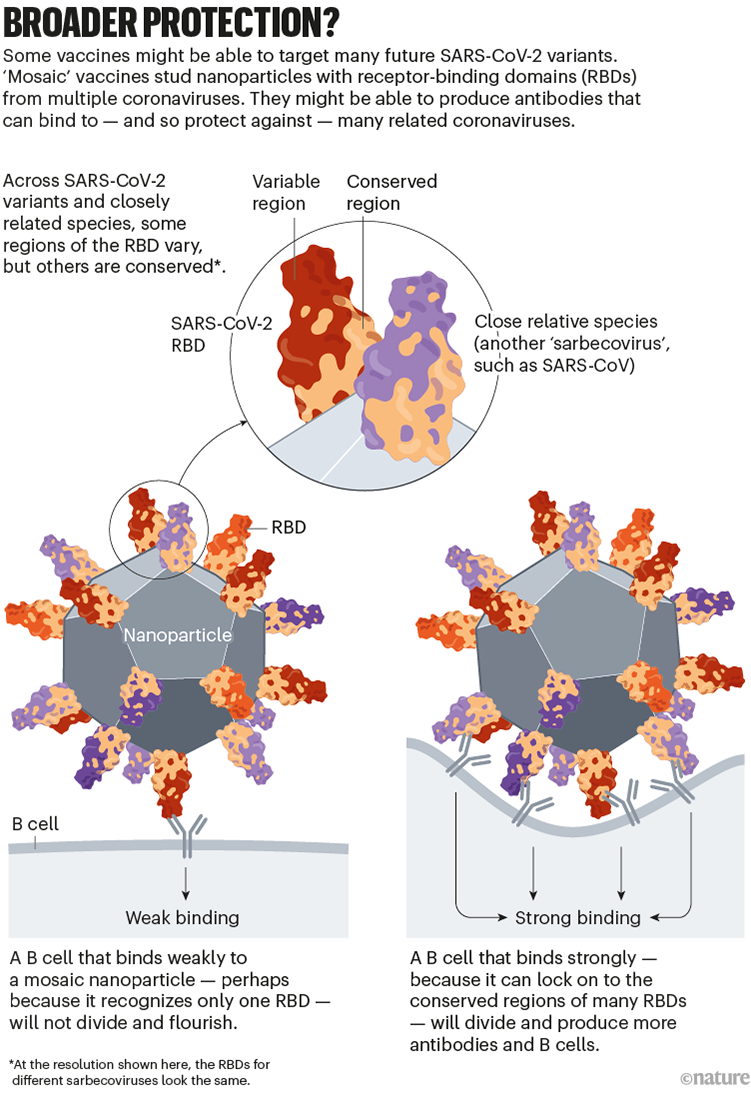
Animal studies suggest that these vaccines do trigger protective responses against diverse sarbecoviruses (see, for example, A. A. Cohen et al. Science 377, eabq0839; 2022). The first clinical trials are set to begin in the next two years.
Going beyond spike
Many first-generation COVID-19 vaccines prompt an immune response only against SARS-CoV-2’s spike protein.
But some next-generation vaccines deliver other viral proteins as well, in the hope of generating a more diverse immune response that safely mimics the protection conferred by infection. This approach could also mitigate the impact of new spike variants (see ‘Targeting other viral proteins’).
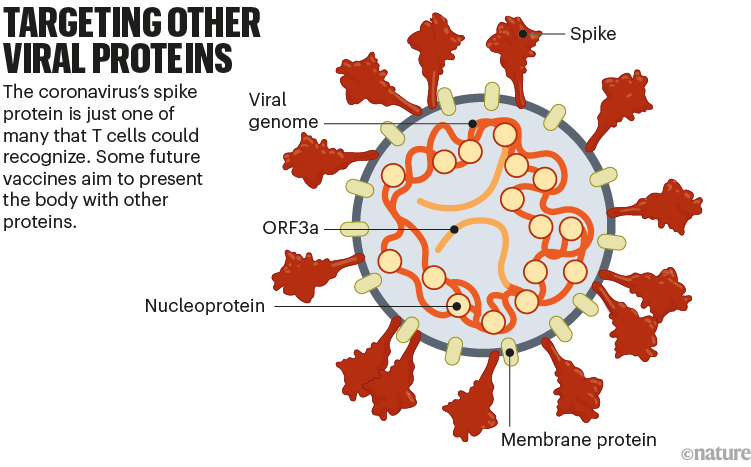
The spike protein is the main target of antibody-making B cells. But T cells that destroy infected cells can recognize many other SARS-CoV-2 proteins. For this reason, vaccines that deliver other proteins could help protect people whose immune systems do not generate strong antibody responses. Such vaccines might also be more resilient to viral evolution, because non-spike proteins tend to vary less between variants.
The US biotechnology company Gritstone is developing one such vaccine: it delivers instructions for several SARS-CoV-2 proteins using mRNA vaccine technology. Meanwhile, Texas biotech company Vaxxinity is developing a protein-based vaccine that would expose the body to multiple antigens. The company says it plans to apply for UK and Australian authorization this year, after a phase III trial showed the vaccine was safe and prompted a strong antibody response when used as a booster.
New platform designs
Another way of categorizing next-generation vaccines is by the method of delivery into the body. Existing vaccines use one of at least four approaches: nucleic-acid vaccines (mostly mRNA) instruct cells to make the SARS-CoV-2 spike protein; inactivated vaccines use versions of the coronavirus itself; protein vaccines are composed of the spike protein or its RBD; and viral-vector vaccines use modified viruses to shuttle instructions for the spike protein into cells. Next-generation vaccines could involve tweaks to these designs or changes to delivery mechanisms that might improve performance.
Self-amplifying RNAs
mRNA vaccines helped to turn the tide of the pandemic, particularly in wealthy countries, where the vast majority of doses have been sold. A twist on this technology might make vaccines cheaper and even more potent, while minimizing side effects.
The vaccines developed by Pfizer–BioNTech and by Moderna (with the US National Institute of Allergy and Infectious Diseases) consist of mRNA instructions for a modified version of spike packaged in a fatty nanoparticle. In an updated version of this technology, self-amplifying RNA (saRNA) vaccines also include instructions for an enzyme that instructs cells to churn out more copies of spike (see ‘Self-amplifying RNA’).
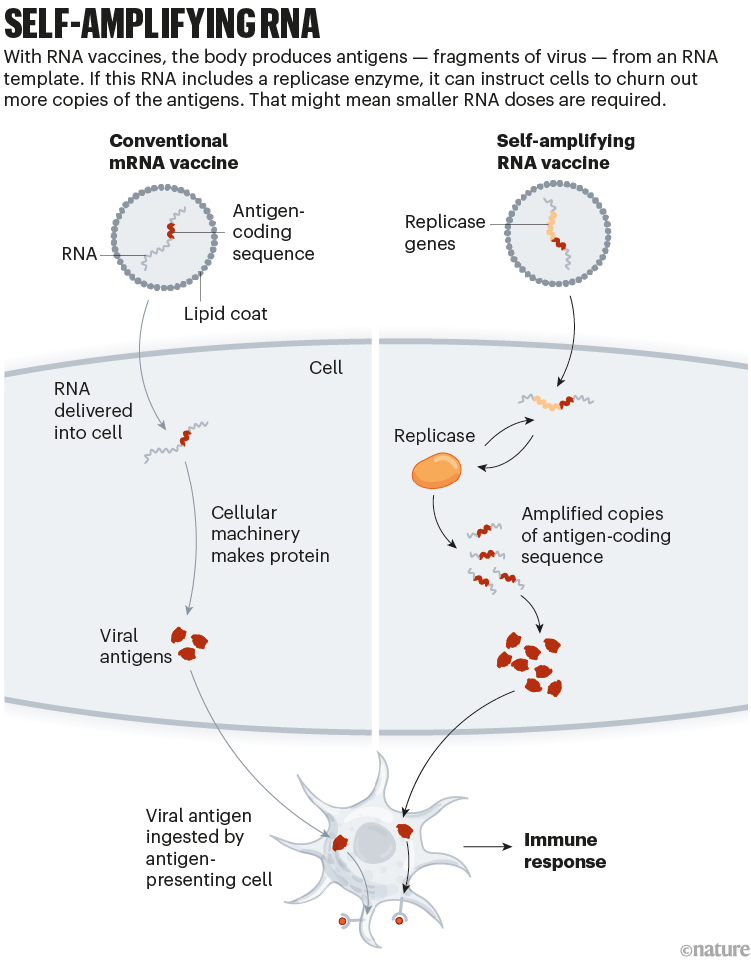
This means that a smaller — and potentially cheaper — dose of saRNA vaccines could achieve the same or even a stronger immune response, compared with conventional mRNA vaccines. A smaller initial dose might also reduce side effects.
One saRNA vaccine, developed by US firm Arcturus Therapeutics, completed a phase III trial in April 2022; the company has now started another phase III trial in Japan that, it says, could lead to an application for authorization there. Gritstone is using saRNA technology to deliver additional SARS-CoV-2 proteins in a candidate T-cell vaccine that has completed a phase I trial.
Proteins on nanoparticles
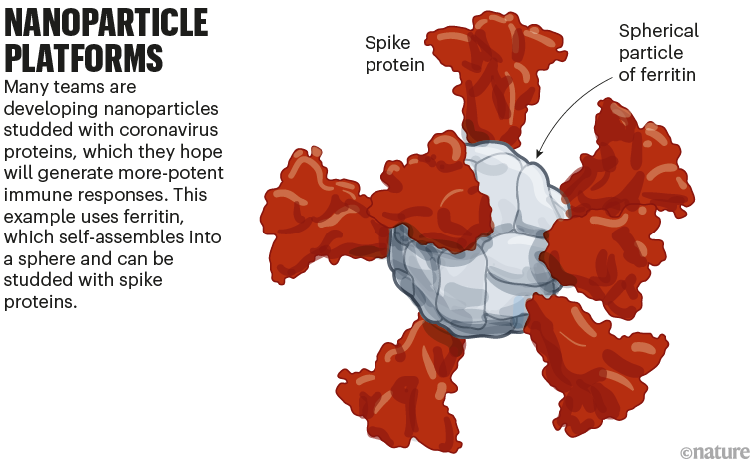
Several protein-based COVID-19 vaccines have been authorized globally, including one made by US biotech firm Novavax. Their low cost and ease of production makes them appealing; they are usually made of stabilized forms of the entire SARS-CoV-2 spike protein or its RBD.
A new class of these vaccines is made of proteins that self-assemble into a soccer-ball-shaped structure, studded with spike or RBD. The repetitive arrangement of the viral molecules, mimicking an actual virus, generates an especially potent immune response.
The ‘mosaic’ vaccines developed by Caltech and the University of Washington (which are studded with RBDs from multiple kinds of coronavirus) are one example of this effort.
Another nanoparticle vaccine has already been approved: in April 2022, South Korean regulators authorized a vaccine, also developed at the University of Washington, containing RBDs from the original version of SARS-CoV-2. A phase III trial showed that the vaccine boosted antibody responses to levels that were several times higher than those generated by the viral-vector vaccine developed by AstraZeneca and the University of Oxford, UK, which uses a chimpanzee adenovirus encoding spike antigens.
However, the South Korean company developing the vaccine, SK biosciences, said in late 2022 that it had paused production amid low demand for the vaccine in South Korea.
A team led by researchers at the US Walter Reed Army Institute of Research in Silver Spring, Maryland, is developing another protein nanoparticle vaccine, using an iron-carrying protein called ferritin. This self-assembles into a spherical particle, and is then studded with the full SARS-CoV-2 spike protein. It is currently being tested in an early-stage trial (see ‘Nanoparticle platforms’).
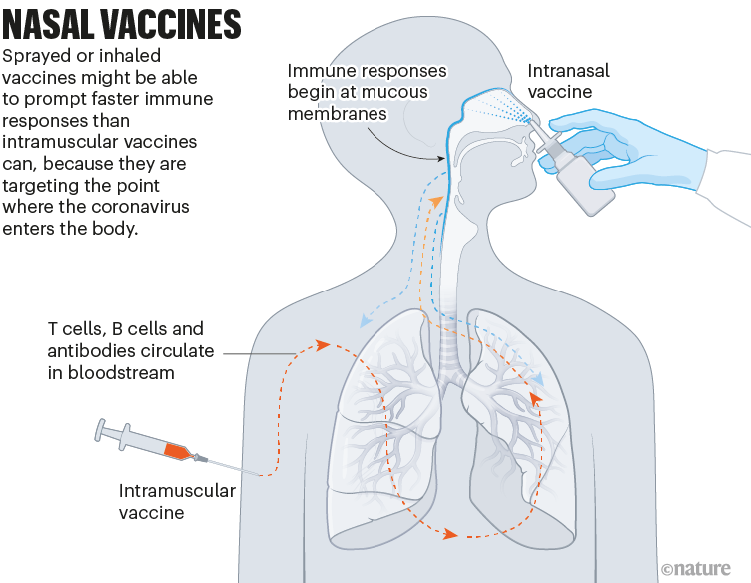
Nasal vaccines
Some COVID-19 vaccines are inhaled as a mist through the nose or mouth, or as nasal drops. By prompting immune responses at the point where SARS-CoV-2 enters the body — in the thin mucous membranes that line the nose and mouth — these vaccines could, in theory, stop the virus before it spreads.
Data from animal studies suggest this might be possible, and at least five nasal vaccines have already been approved for use — two in China and one each in India, Iran and Russia. But there are no data yet on whether these vaccines are better than injections at cutting down infection or transmission of the virus (see ‘Nasal vaccines’).
A key challenge to the development of these and other next-generation COVID-19 vaccines is proving that they offer genuine improvements over existing jabs, says Melanie Saville, executive director of vaccine research and development at the Coalition for Epidemic Preparedness Innovations (CEPI), an Oslo-based foundation that is a leading funder of next-generation COVID-19 vaccines.
Stiff competition
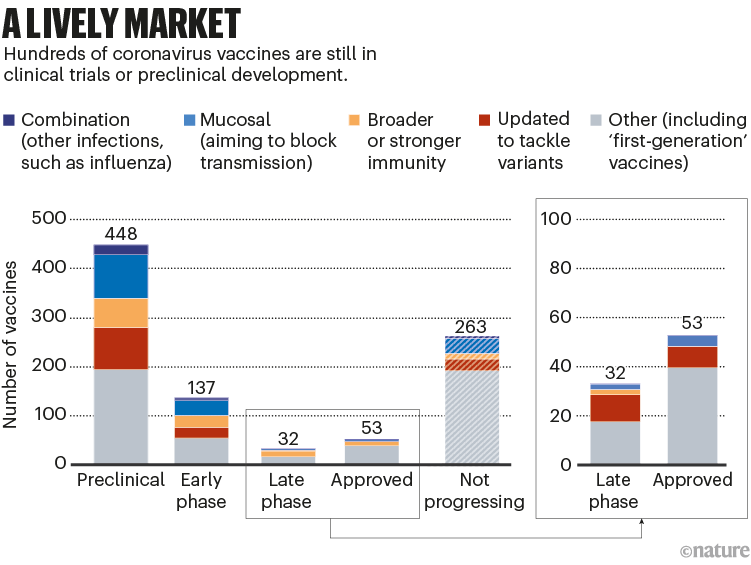
All the next-generation vaccines will have to fight for market share. More than 50 vaccines have already been approved, and there are hundreds in early- and late-stage clinical trials; hundreds more have been abandoned (see ‘A lively market’).
Among the approved vaccines, just a few dominate the doses that have been administered (see ‘Leading players’).
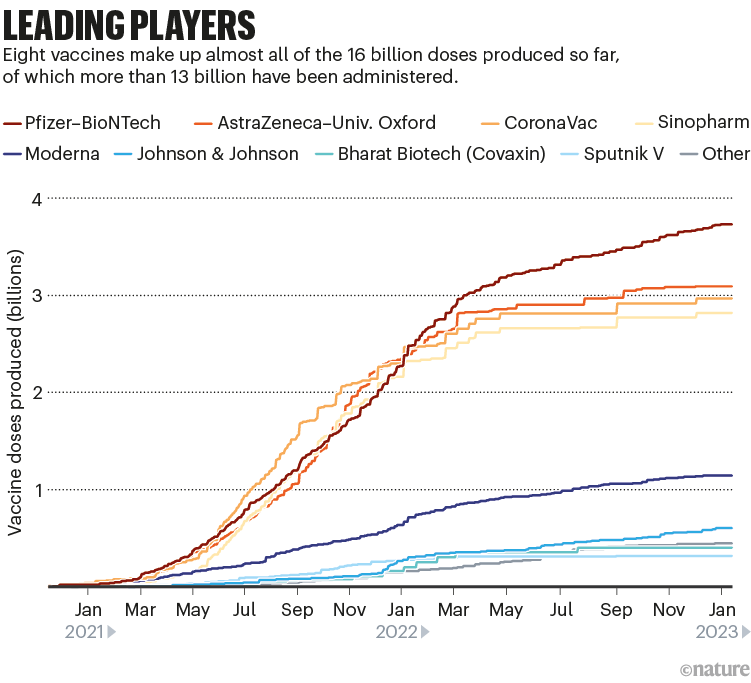
Despite the flurry of research, current mRNA jabs such as the Moderna and Pfizer–BioNTech ones are likely to hold sway, says Matt Linley, analytics director at Airfinity, a life-sciences information firm in London. The fast development of bivalent vaccines that included an Omicron component showed that these vaccines could be adapted quickly. If another update is needed, “mRNA vaccinations would be market leaders in being able to react quickly”, says Linley.
COVID-19 is no longer seen as the all-encompassing emergency it once was, and so developers and regulators will move more slowly compared with the breakneck pace of the first-generation vaccines, adds Saville. “We shouldn’t rush these through, because these need to be the types of vaccine that will be durable in the long term for COVID-19.”
But even if work on new vaccine technologies doesn’t directly pay off against COVID-19, it could still support efforts to combat other diseases, Saville says, such as CEPI’s work on a ‘vaccine library’ for different virus families to improve preparation for future threats.
COVID-19 - Latest - Google News
February 01, 2023 at 05:12PM
https://ift.tt/LqVgwzN
The next generation of coronavirus vaccines: a graphical guide - Nature.com
COVID-19 - Latest - Google News
https://ift.tt/sB8NAoY
Bagikan Berita Ini














0 Response to "The next generation of coronavirus vaccines: a graphical guide - Nature.com"
Post a Comment